How Does Density of Gas Depend on Temperature
When the temperature decrease density. Find step-by-step Chemistry solutions and your answer to the following textbook question.

Gas Density An Overview Sciencedirect Topics
The higher the temperature the more the molecules are spread out and the lower the density as shown in the graphic on the left.
. P R d T v Where T. Its invested proportional to volume so that means that its pure crisp. View chapter Shortcuts Tips.
100 5 ratings for this solution. When the temperature becomes warmer the density of a gas decreases. EXAMPLES At 10 C 10000.
We know that for gases the volume is directly proportional to temperature by the equation PVnRT. According to ideal gas equation P V n R T P m M V R T d R T M so densityd of a gas depends up on its molar mass pressure and temperature. Calculation of Density of an Ideal Gas.
To me density is the amount of particles how they are moving and how they are packed in something Density. Problem solving tips. P FA force per unit area Hydrostatic equation.
How does it depend on the molar mass of the gas. View solution View more. Density and Temperature Relationship.
The density is defined as the ratio of the mass to the volume. The density of a gas depends up on its molar mass pressure and temperature. For a given amount of a gas how does the mean free path of a gas depend on a density b temperature at constant volume c pressure at constant temperature d volume at constant temperature and e size of the atoms.
Increase in density and vice versa. First week only 499. Volume decreases depressing body and will leave J R.
Start your trial now. As temperature increases the density of liquids and gases decreases. I make MP and are constants so I have my densities and factually product pressure.
Density is an important parameter when performing calculations dealing with heat and mass transfer. For a gas the density is directly proportional to the pressure and inversely proportional to the temperature absolute ie Kelvin. Since volume is in the denominator increasing the volume decreases the density.
An increase or decrease in temperature causes the expansion or contraction. The density will decrease as pressure increases The density will increase as pressure increases The density does not. The density and temperature relation are proportionate.
How does the density of a gas depend on the molar mass of the gas. Explain the relationship of density and pressure. Remember that temperature is related to the average kinetic energy of the atoms or molecules within the substance.
If there is any proportion that them so is the density is in fronting for another 10. Density ρ Mass mVolume V where m mass of the body. Density of an object depends on the temperature.
P - g z Transformation of temperature T K T C 27315 T F T C x 95 32 Ideal gas law For dry air. As temperature increases the molecules in liquids and gases move more quickly which makes them collide with one another more often. The density will increase as pressure increases.
Solution for How does the density of a gas depend on temperature. Density is mass divided by volume. The density will increase as the molar mass of the gas increases.
Density is the amount of mass per unit of volume. It is also used to derive many important dimensionless numbers such as. Gas Density examples based upon differences in temperature.
How does the density of a gas depend on temperature. View solution The vapour density of a gas is 224. It depends on density and temperature.
Density changes with temperature because volume changes with temperature. As temperature decreases the density increases. Within a particular phase how does the density depend on temperature.
This causes the molecules to spread apart which. No so far for pressure. Which means for unit volume-When density increases the temperature decrease.
Examples of the use of the figures are given. In terms of a gas density and pressure have a proportional relationship. No question it means the ion intensity to lower the temperature on the lower the better.
Weve got the study and writing resources you need for your assignments. Now lets go to pressure right here. In physics specific gravity or relative density is another term used to define the density of a substance relative to water.
O The density does not depend on the temperature changes O The density will increase as temperature increases O The density will decrease as temperature increases Submit Request Answer Part B How does the density of a gas depend on pressure. MV mass per unit volume Pressure. Correlations for fuel oils density and temperature are calculated by use of tools based on ASTM D 1250-04 and IP 20004 API Manual of Petroleum Measurement Standards Chapter 11- physical properties Data Section 1Temperature and pressure volume correction factors for generalised crude oils refined products and lubricating oils.
The density of gases depends upon the temperature. The density of an ideal gas depends on the molecular weight temperature and pressure of the system. When density decreases temperature increases.
The density of the gas at S T P is. V volume occupied by the body. When more temperature increases density reduces.
How does the density of a gas depend on pressure. So when you do Chris temperature volume decreases and necessity Kruses so that explains how temperature affect the density off gas. The density will decrease as temperature increases.
P R d T R d 287 JkgK For moist air. The result is that warm gases rise and cool gases sink. That is the density is inversely proportional to temperature.
Density massvolume As you heat something up the volume usually increases because the faster moving molecules are further apart. The quantity of something per unit volume unit areaor unit length Densitymassvolume Differences in temperature in Earths solidliquid mantle and lithosphere cause differences in density that. In other words the higher the temperature the lower the density.
Now how does their density Gas depends on.

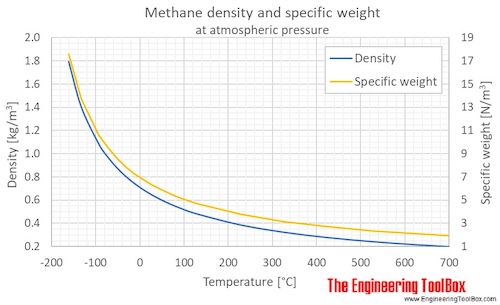
Methane Density And Specific Weight Vs Temperature And Pressure
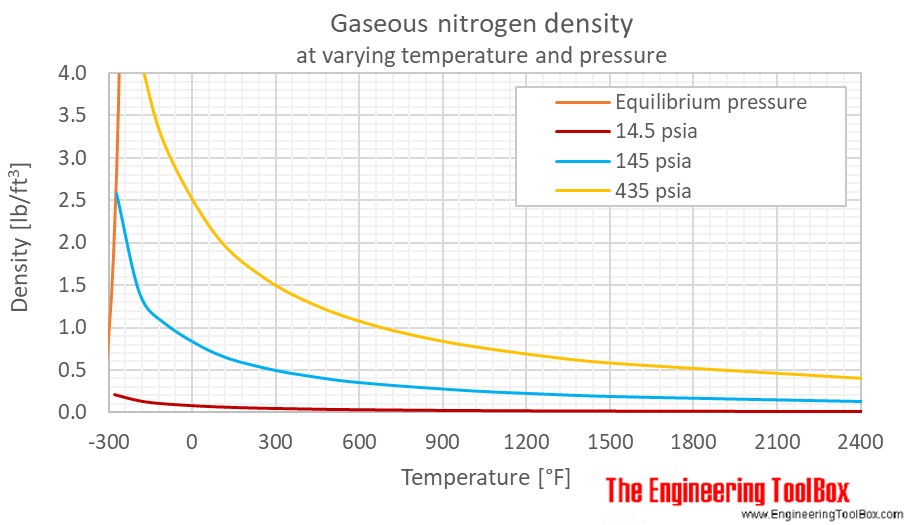
Nitrogen Density And Specific Weight Vs Temperature And Pressure

Nitrogen Density And Specific Weight Vs Temperature And Pressure
No comments for "How Does Density of Gas Depend on Temperature"
Post a Comment